Company Profile
About Bionime
Bionime was established in April 2003 with the goal of improving the lives of people with diabetes through better self-monitoring. Bionime is focused on designing and manufacturing accurate medical testing equipment. Our first product line started out with accurate blood glucose monitors that mimic the capabilities state-of-the-art devices usually found in healthcare research facilities. Our product line has since expanded into multiple different Self-Monitoring Blood Glucose (SMBG) Systems.
With the integration of top-tier scientific expertise, Bionime has successfully commercialized many patented technologies. This includes our unique Validus technology test strips which enables patients to receive accurate readings on-the-go.
Today Bionime operates in most major markets including Europe, the Middle East, Africa, the Americas, and Asia. Our products have received outstanding international recognition in clinical tests and by well-known journals. Our products continue to be distributed worldwide with great success.
Vision
With our “peace of mind“ medical devices, we offer solutions to patients to help them accurately manage and control their own health.
Philosophy
Better health for more people
Our philosophy is to provide products of the highest quality both to people in need and their caregivers to help them easily manage their day-to-day health. With this foundation in mind, Bionime has successfully integrated different technologies, combining Swiss designs and made-in-Taiwan quality assurance, to provide a continuous supply of world-class diagnostic solutions to our customers around the world. Our unique product designs provide accurate, precise test results, deliver a superb user experience, build customer satisfaction, foster trust, and ultimately help us fulfil our vision of better health for more people. With this philosophy in mind, we will continue striving to ensure good health and a quality life is possible for everyone.
Corporate Milestones
Bionime together with qualified experts in the field of diabetes have initiated and completed several SMBG patents during the last few years. Nevertheless, innovation and creativity continue to characterize Bionime's efforts in the SMBG field.- May
- Launches GM700 Pro 2 for easy data management and synchronization within hospitals
- Feb
- Bionime Singapore established
- Feb
- Bionime Indonesia established
- Nov
- Awarded the Taiwan Excellence Award (Rightest Blood Glucose Ecosystem)
- Nov
- Rightest CARE Total Solution System has won the 2019 Taiwan Excellence Award
- Mar
- Established Malaysian subsidiary - Bionime (Malaysia) SDN BHD
- Feb
- Acquired ISO 27001:2013 information security management system certification
- Oct
- Meets ISO 13485:2016 certification of quality management systems for medical devices.
- July
- Launches Rightest CARE App, the blood glucose self-management App in Google Play and App Store.
- July
- GM700SB Bluetooth glucometer has been first released in Taiwan.
- June
- Releases new Rightest logo

- Feb
- GM700 Pro has won the Taiwan Excellence Award.
- Aug
- Certified by Brazil's National Health Surveillance Agency and received ANVISA Certificate
- Apr
- Successfully obtained Good Manufacturing Practices (GMP) certification from the Ministry of Health and Welfare, Republic of China (Taiwan) for additional products including uric acid and cholesterol detections.
- Dec
- Received capital injection by private placement of Dongbao Pharmaceutical Co., Ltd.
- Apr
- Obtained new patent in China is regarding "Insulin measuring pen with biological sensors".
- Jan
- Established subsidiary in Pingtan, China
- Dec
- Received new patent in Germany for “Measuring Device for an Analyte”.
- Jun
- Members of the Diplomatic and Economic Representatives in Taiwan Visit Bionime for Exchange International Business and Bionime’s Competitive Advantages
- Jan
- GM720 won the Taiwan Excellence Award
- Oct
- GM720 won the National Industrial Innovation Award
- Sep
- GD500 won National Invention and Creation Award
- Sep
- Pass Taiwan GMP certification
- Mar
- Mylife Unio Won Reddot Design Award
- Mar
- New Building & Factory completion
- Dec
- New Product “Mylife Unio” shipped to European Market
- Jan
- GE Selects Bionime to Launch Innovative Diabetes Solutions
- Dec
- Bionime acquires the Innovation Award from Taiwan Ministry Economic Affair.
- Sep
- BIONIME has passed certification of OHSAS18001.
- Jul
- Acquisition land property for extending operation
- Jul
- Pass Taiwan AEO Certification
- Mar
- Strategic global cooperation with GE during term of Licensing Agreement in diabetes technology.
- Dec
- Become a public company and traded officially as a hi-tech company on the TWSE.
- Sep
- Granted the highest award of Taipei Biotech competition.
- Aug
- Approved to apply a high-tech list company by Industrial Development Bureau, Ministry of Economic Affairs.
- Feb
- Set up Bionime Australia Pty Limited.
- Jul
- Granted SFDA of RightestGM100, 110.
- May
- Launch of Rightest GM550 in Europe.
- Apr
- Pass KGMP audit.
- Jan
- Listed on Emerging Stock Market (#4737)
- Oct
- In strategic alliance with Ypsomed, a Switzerland-based solution provider of injection system for diabetes treatment.
- Sep
- Granted FDA 510(K) of Rightest GM100, 110.
- Aug
- First shipment of ODM products.
- Mar
- Launch new Lancing Device to the market.
- Feb
- Set up BIONIME USA Corporation.
- Aug
- Passed ISO 13485 and CMDCAS audit.
- July
- Passed TNO Certification.
- Mar
- Launch Rightest GM100.
- Feb
- Set up BIONIME (ShenZhen) Co., Ltd.
- Aug
- Granted China SFDA of Rightest GM300 Aug.
- Jun
- Passed Taiwan GMP Certification Jun.
- May
- Launch Rightest GM300
- Apr
- Set up BIONIME GmbH Switzerland in Apr.
- Jan
- Granted FDA 510(K) of Rightest GM300.
- Dec
- Set up Bionime Corporation.
- Nov
- Granted CE Certification and passed ISO13485 Certification.
- Oct
- The development of the first generation of blood glucose meter was completed.
- Apr
- BIONIME was founded at Dali City, Taichung County.
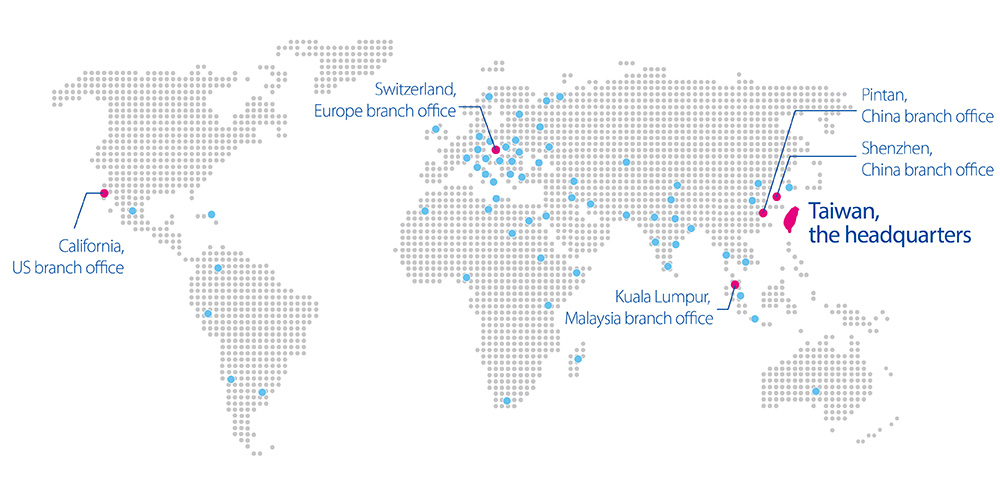
BIONIME CORPORATION
- TEL:+886 4 2369 2388
- FAX:+886 4 2261 7586
- Address:No.100, Sec. 2, Daqing St., South Dist., Taichung City 40242, Taiwan (R.O.C.)
- E-mail:info@bionime.com
- Toll-Free:0800 371 688
- Service Hours:Mon - Fri, 8:30 am - 5:30 pm
Bionime USA Corporation
- TEL:+1 909 781 6969
- FAX:+1 909 781 6970
- E-mail:info@bionime.com
- Address:1450 E Spruce Street, Bldg. B, Ontario CA 91761-8314
- Website:www.bionimeusa.com
Bionime (Malaysia) SDN BHD
- TEL:+603 8322 8213
- E-mail:enquiry@bionime.com.my
- Address:C-05-11, iTech Tower, Jalan Impact, Cyber 6, 63000 Cyberjaya, Selangor, Malaysia
- Website:www.bionime.com.my
Bionime (Pingtan) Co., Ltd.
- TEL:+86 591 6252 3167
- FAX:+86 591 6252 3166
- E-mail:info@bionime.com
- Address:Taiwan Pioneer Park, Pingtan Comprehensive Experimental Area, Pingtan County, Fuzhou City, Fujian Province, China
Bionime (Shenzhen) Co., Ltd.
- TEL:+86 400 780 6098
- E-mail:info.cn@rightest.com.cn
- Address:Room 1008, Building 7, Xiangyu Cross-Strait Trade Center, Kunshan Development Zone, Suzhou
- Website:www.rightest.com.cn